 The Whitney Laboratory for Marine Bioscience
The Whitney Laboratory for Marine Bioscience

Dr. Barbara-Anne Battelle graduated with a B.A. in biology, chemistry and German from Skidmore College in Saratoga Springs, New York. She earned her Ph.D. in biology from Syracuse University. As a postdoctoral fellow, Battelle worked in the Department of Neuropathology and the Department of Neurobiology at Harvard Medical School. She then became a Senior Staff Fellow at the National Eye Institute at NIH in Bethesda, Maryland.
In 2007 she received the Howard Hughes Medical Institute Distinguished Mentor Award.
Email: battelle@whitney.ufl.edu

It may be hard to believe, but on a dark moonless night, our eyes are actually up to 10,000 times more sensitive to light than they are during the day time. This change in sensitivity allows us to make the most of the limited light available at night without being blinded during the day. It is regulated in part by cyclical 24 hour “clocks” that are embedded in the light sensing tissue in our eyes, the retina. These clocks cause biochemical changes in the retina that increase light sensitivity when it is dark and decrease it when it is light. Most people notice as they age their vision changes, usually for the worse and especially at night.
Retinas and photoreceptors change their function during day and night. In order for the full range of day to night fluctuations in visual function to occur, two things are required: rhythmic changes in environmental light and signals from internal circadian clocks. We seek to understand the biochemical mechanisms by which light and clock input, separately and together, regulate retinal and photoreceptor functions. Through these studies we are gaining a more complete understanding of the requirements for normal vision.
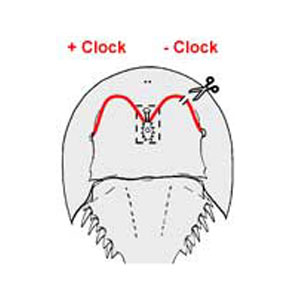
The horseshoe crab Limulus polyphemus, offers a number of advantages for these studies. The influence of the circadian clock on the structure and function of Limulus photoreceptors is robust and well characterized. In addition, the unique circadian organization of the Limulus visual system permits easy experimental manipulation of the circadian input to the eyes. Specifically, the circadian clock that drives changes in visual function is located in the brain, and circadian signals reach the eyes via well-characterized efferent neuronal projections through the optic nerves. The efferent projection to the lateral eye can be conveniently cut to produce an eye lacking circadian efferent input while the circadian input to the other eye (Figure 1), and the circadian biology of the animal as a whole, is normal.
Figure 1. Diagram of the dorsal side of a horseshoe crab showing the projections of the lateral optic nerves from the brain to the lateral compound eyes. These optic nerves contain axons of neurons that are driven by a central circadian clock located in the brain. The brain is diagramed within the boxed area. The optic nerves are in red. The scissors and the break in the optic nerve on the right indicates that the optic nerve has been cut, and thus the eye on the right receives no clock input while clock input to the left eye is normal.
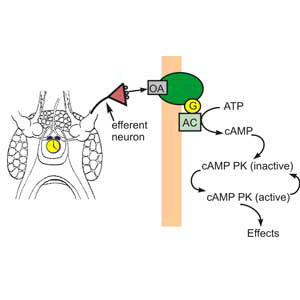
What are the functional consequences for photoreceptors of the nighttime clock-stimulated phosphorylation of a major photoreceptor protein? Much evidence from this lab and others indicates that circadian changes in Limulus photoreceptors are brought about by the release of the biogenic amine octopamine from the terminals clock-driven efferent neurons that project to the eyes, a subsequent rise in cAMP in photoreceptors and activation of cAMP-dependent protein kinase (PKA). Thus some clock-driven changes in photoreceptor function must involve the phosphorylation of specific proteins. (Figure 2)
Figure 2. Model of the biochemical cascade responsible for many clock-driven changes in Limulus retinal functions. From: Dalal and Battelle Current Zoology, 2010.
A particularly interesting clock-regulated phosphoprotein we identified is Limulus myosin XXI, the Limulus homologue of the Drosophila ninaC gene products which are required for normal photoreceptor function in Drosophila.
Class XXI myosins are unconventional myosins in that have a kinase domain at their N-terminus, and as with most unconventional myosins, the functions of class XXI myosins are not known. Similar kinase-myosins are expressed in vertebrate photoreceptors and hair cells.
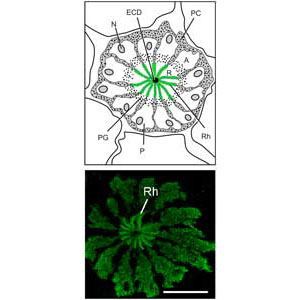
Figure 3. TOP: Diagram of a cross section of one ommatidium in the Limulus lateral eye. Photoreceptors (P) are arranged like the sections of an orange. At the very center of the structure is the dendrite of the eccentric cell (ECD), a second order neuron electrically coupled to the photoreceptors. The green rays represent the rays of the photosensitive membranes of rhabdomeres (Rh). In this diagram, pigment granules in the photoreceptors (PG) separate the separate the photosensitive rhabdomeral segment (R) of the photoreceptor from its photoinsensitive segment or arhabdomeral segment (A). Photoreceptor nuclei are in the A segment. Pigment cells (PC) surround the photoreceptors.
BOTTOM: Confocal image showing the distribution of myosin XXI in lateral eye photoreceptors. It is distributed throughout the photoreceptors and concentrates over the rays of the rhabdom. Scale bar = 25um. From Dalal and Battelle, Current Zoology, 2010.
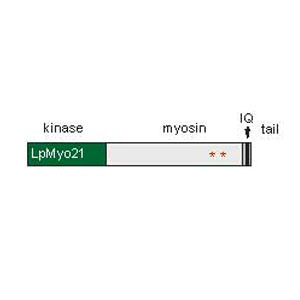
Major goals of our current research are to understand the functions of Limulus myosin XXI, how these functions are modified by phosphorylation, and how Limulus Myosin XXI phosphorylation influences photoreceptor function.
Figure 4. Model of myosin XXI. Its kinase domain at the N-terminus is in green, its myosin-like domain in light gray, its tail domain in dark gray. Its single IQ calmodulin binding domain is in black. Two clock-regulated phosphorylation sites represented by red stars.
Does the clock influence dark-adaptive changes in photoreceptor biochemistry? In both vertebrates and invertebrates long-term light and dark-adaptation involves the translocation of proteins into and out of the photosensitive compartment of photoreceptors. The rhabdomeral level of one visual pigment opsin 1 is low during the day and high during the night. The rhabdomeral level of Gq, a protein activated by photoactivated rhodopsin, is also low during the day and is high during the night, while the rhabdomeral level of arrestin, a protein that stops phototransduction, is high during the day and low during the night. Light is a major driving force for these observed changes in proteins levels at the rhabdom. Ongoing studies are testing the hypothesis that at least some of these changes are regulated by signals from circadian clocks.
What are the functional consequences of the differential regulation of opsin co-expression in Limulus photoreceptors? Until relatively recently it had been thought that an individual photoreceptor expresses only one type of visual pigment or opsin. However, recent studies have revealed a growing number of examples of photoreceptors in both vertebrates and invertebrates that express more than one opsin. The functional relevance of opsin co-expression is still largely unknown.
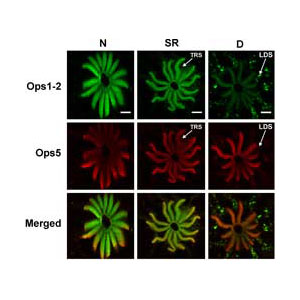
We recently found that Limulus photoreceptors express at least two different types of opsins, Opsin 1-2 and opsin 5. The relative levels of these opsins in the photosensitive membrane of photoreceptors change with a diurnal rhythm (See the figure above) and that their expression in the photosensitive membrane is regulated differently by light and by the animal’s circadian clock. A comparison of the amino acid sequences of these opsins indicates they may have different spectral sensitivities. Ongoing studies are examining the function of the newly identified opsin and the functional consequences in photoreceptors for the differential regulation of their expression.
Confocal images of lateral eye rhabdomeres stained for opsin1 (green) and opsin 5 (red). The level of opsin1 in the rhabdom is dramatically higher at night compared to day. The level of opsin 5 is unchanged day to night. From Katti et al., Journal of Experimental Biology, 2010.
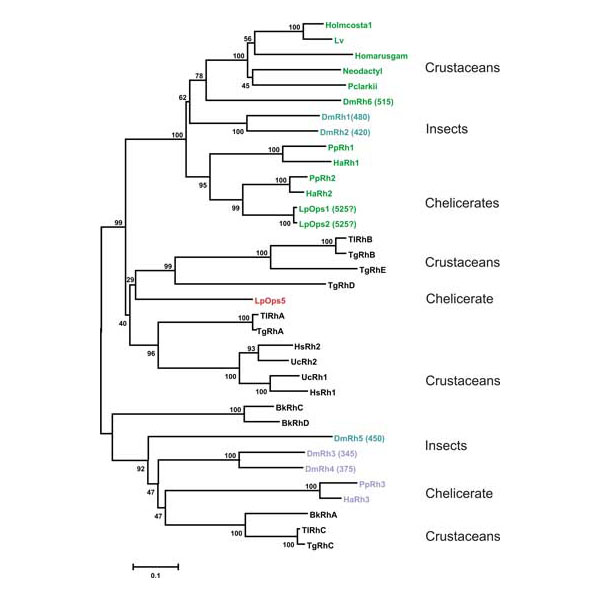
Transcriptome analysis of Limulus photoreceptors. In collaborative studies with the Moroz laboratory at the Whitney Laboratory and the Oakley laboratory at UC Santa Barbara, we are examining the transcriptomes of Limulus ventral photoreceptors and lateral eye retinas. These studies are opening avenues of research that will provide new insights into photoreceptor function and evolution. Such analysis resulted in the identification Limulus 5.
Phylogenetic analysis of the relationships between Limulus opsins and other arthropod opsins. Limulus opsins 1 and 2 cluster with blue-green opsins from other chelicerates. Opsin 5, on the other hand, clusters with opsins previously thought to be unique to crustaceans. The spectral sensitivities of these opsins are not yet known.
From Katti et al., Journal of Experimental Biology, 2010.